
Exposure to Vinyl Chloride Monomer and Combustion Products in a Train Derailment
In what ways can emergency responders be affected by the exposure?
- By Bernard Fontaine
- Mar 07, 2023
Over 2.5 million emergency responders in the U.S. face hazardous exposures while on duty [NIOSH 2013a], and this population has been identified as having the greatest risk of exposure during a disaster [Pak et al. 2008]. Responders to a chemical release following a train derailment must immediately assess the risks by gathering information about the situation, analyzing available options and taking action to implement decisions to protect themselves, the public, other responders and the environment [NIOSH 2004]. According to the U.S. Department of Transportation Bureau of Transportation Statistics, there have been a total of 54,570 freight and passenger train derailments, averaging 1,705 each year between 1990 and 2021.
There have been many questions about whether the burning of the vinyl chloride monomer (VCM) tank cars in the recent train derailment in East Palestine, Ohio, could have resulted in the formation of dioxins and furans. Let’s look at the evidence to date. Of the 38 rail cars derailed, 11 tank cars contained hazardous materials including VCM. One of the VCM tank cars was reportedly leaking. The VCM boiling point is −13.4 degrees Celsius (7.9 degrees Fahrenheit), with a vapor pressure of 2580 mmHg at 20 degrees Celsius (68 degrees Fahrenheit).
Fire impingement to the VCM tank car exterior caused the liquid temperature to rise near the boiling point. In order to prevent a Boiling Liquid Expanding Vapor Explosion (BLEVE), crews began a controlled release of the liquid VCM. VCM was released from five rail cars into a trough that was then ignited, creating a large plume of black smoke over the village of East Palestine. After completing the controlled release, crews began the "wrecking" process, in which the empty tank cars were moved off the tracks and relocated to a safe area nearby. Based on the evidence, could the controlled burn have released hazardous combustion products like PCDDs and PCDFs into the air? Well, here is what we know to date.
East Palestine was not the only recorded railroad incident involving VCM. On Nov. 30, 2012, a train transporting VCM derailed while a bridge collapsed while crossing over Mantua Creek, in Paulsboro, New Jersey, near Philadelphia. Four rail tank cars fell into the creek, breaching one tank and releasing about 23,000 gallons of VCM into the waterway. There was no fire associated with the mishap, but workers and nearby residents were exposed to VCM.
In this case, the National Oceanic and Atmospheric Administration (NOAA) Office of Response and Restoration (OR&R) provided guidance to help local and federal authorities address early concerns about the possible health effects, evacuation decisions, proper protective equipment for responders, impacts to the nearby Philadelphia airport and reactivity between VCM and another rail tank car containing ethyl alcohol. OR&R developed software products responders use to address these issues: ALOHA, an air dispersion model, and CAMEO Chemicals, a hazardous material database.
Responders were categorized by profession, including Emergency Medical Services, firefighters, police officers, and hazardous material technicians. Because a typical work shift lasts 12 hours, participants were categorized by the duration of exposure: those who worked a total of less than 12 hours and those who worked more than 12 hours in the evacuation zone throughout the entire eight-day period.
Removing the derailed train cars proved to be a logistically complicated process. First, the Coast Guard coordinated the removal of the last 600 gallons of vinyl chloride from the breached tank by using acetone and suctioning out the vapors before attempting to move the tank. Next, the response team successfully brought in cranes and barges to remove the rail cars from Mantua Creek, re-establish them on the rail and have them transported away from the site. Because of the acute health effects associated with VCM, the New Jersey Department of Health requested the assistance of the Agency for Toxic Substance and Disease Registry and CDC to assess the health of the neighboring community and emergency responders.
Symptoms were grouped according to clinical presentation. Neurological symptoms included dizziness, weakness and loss of balance. Upper respiratory symptoms included a runny nose, burning nose or throat and hoarseness, whereas lower respiratory symptoms included shortness of breath, chest tightness, wheezing and burning lungs. Coughing, increased congestion and increased phlegm are presented separately from other respiratory indicators because their cause could be upper or lower respiratory in nature.
A survey of the 93 emergency responders reported headaches (26 percent), upper respiratory tract symptoms (26 percent) and lower respiratory tract symptoms (22 percent) during the response. Only 22 percent of the emergency responders wore a respirator during the incident. Twenty-one respondents sought a medical evaluation. CDC suggested that response agencies implement the Emergency Responder Health Monitoring and Surveillance system for ongoing health monitoring of the emergency responders, especially when respiratory protection is needed for situations with unknown exposure levels or known exposure levels above an established occupational exposure level. Eighty percent (80 percent) of respondents reported that they received training in first responder awareness, 52 percent in first responder operations, 27 percent in hazardous materials for technicians and 27 percent in hazardous waste operations and emergency response.
A paper written by O’Mara et. al. in the American Industrial Hygiene Journal described the combustion products from VCM. A variety of analytical techniques were used to evaluate the combustion profile of VCM. The profile included various flame temperatures, soot content and a combustion gas analysis. Depending on the amount of VCM-air premixing prior to combustion, the temperature of a VCM flame ranges from 950 to 1466 degrees Celsius.
Similarly, the soot or unburned carbon content of a VCM flame varied from three to six weight percent. An analysis of the combustion gases from VCM reveals the following composition: HCl 27,000 ppm, CO2 58,100 ppm, CO 9,500 ppm, phosgene 40 ppm and VCM trace. From a hazard standpoint, the gross quantity of hydrogen chloride in the air is the main source of danger in a VCM fire. The research study did not look at the potential of forming dioxins or furans as combustion products.
Ambient air concentrations of VCM are generally quite low in the ambient environment, with exposure occurring from the discharge of exhaust gases from factories that manufacture or process VCM, tobacco smoke or evaporation from areas where chemical wastes are stored. Could the controlled burn of VCM contribute to exposure to dioxins and furans? What is the acute and chronic effect of VCM exposure? Well, let’s look further into the evidence.
OSHA regulates exposure to VCM but does not cover exposure to railroad workers since they are covered by the Federal Railroad Administration. However, hazardous material responders hired by the railroad are covered by OSHA. The OSHA VCM standard is 29 CFR 1910.1017 for the general industry to protect workers' manufacture, reaction, packaging, repackaging, storage, handling or use of VCM. It also covers any hazardous operation, meaning any operation, procedure or activity where a release of either VCM liquid or gas might be expected as a consequence of the operation or because of a mishap in the operation, which results in employee exposure in excess of the OSHA Permissible Exposure Limit.
Acute exposure of humans to high levels of vinyl chloride via inhalation has resulted in effects on the Central Nervous System (CNS), such as dizziness, drowsiness, headaches and giddiness. The American Industrial Hygiene Association published several emergency response planning guidelines, such as ERPG-1/ERPG-2/ERPG-3, which allow adequately protected workers to be exposed to airborne concentrations of 500 parts per million (ppm)/5,000 ppm/20,000 ppm depending on the location to the source of the exposure. The OSHA ceiling concentration without a respirator is a maximum of 5 ppm for up to 15 minutes. The eight-hour Time-Weighted Average exposure is 1 ppm in the air. The National Institute for Occupational Safety and Health (NIOSH) does not have any acceptable exposure criteria since VCM is considered a potential human carcinogen by causing liver angiosarcomas.
Symptoms of chronic exposure include lassitude (weakness, exhaustion), abdominal pain, gastrointestinal bleeding, enlarged liver, pallor or cyanosis of extremities and Raynaud's phenomenon (fingers blanch and numbness and discomfort experienced upon cold exposure), and contact with liquid VCM can cause frostbite. CNS effects from chronic exposures include dizziness, drowsiness, fatigue, headache, visual and/or hearing disturbances, memory loss and sleep disturbances. Peripheral nervous system symptoms (peripheral neuropathy, tingling, numbness and weakness) have been reported in workers exposed to VCM.
Figure 1 – Health Data from Inhalation Exposure
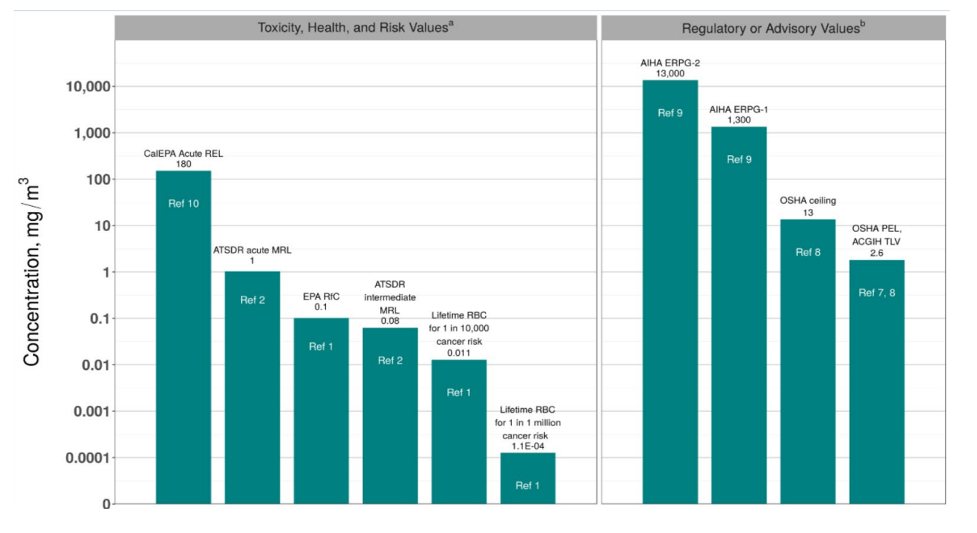
Source: Vinyl Chloride March 26, 2020 - US EPA
Several epidemiological studies reported an association between VCM exposure in pregnant women and an increased incidence of birth defects; however, other studies have not supported these findings. Several case reports involving worker exposures suggest that male sexual performance may be affected by VCM; however, these reports are limited by possible concomitant exposure to other chemicals and lack of exposure estimates. Testicular damage and decreased male fertility have been reported in rats exposed to VCM.
Flame chemistry in incineration systems involves the formation of many organic products of incomplete combustion, including chlorinated species such as polychlorinated dibenzo-p-dioxins (PCDD) and polychlorinated dibenzofurans (PCDF). There are two temperature windows in which they can form: the “homogeneous” route between 500 and 800 degrees Celsius and the “heterogeneous” one at 200 to 400 degrees Celsius.
Homogeneous reactions are the result of the pyrolytic rearrangement of chlorinated precursors such as chlorophenols and chlorobenzenes in the gas phase, which have not been researched as extensively as the heterogeneous mechanism. The heterogeneous formation is a catalyzed reaction that takes place on the ash or soot particles present in combustion systems. The formation process is driven by oxidation, and the rate is related to carbon burn-off. Dichlorination and decomposition proceed at elevated temperatures. The reaction appears to take place on the external surface of the particles based on the content of carbon and chlorine. Different mechanisms are postulated for PCDD and PCDF.
PCDDs and PCDFs are a class of polyhalogenated aromatic hydrocarbons that all share a similar chemical structure. Dioxins, in their purest form, look like crystals or colorless solids. Most dioxins and furans are not man-made or produced intentionally and do not serve any useful purpose. They are formed as by-products of numerous industrial activities and combustion processes. PCDDs and PCDFs are created when halogenated organic chemicals are subjected to high-temperature contaminating exhaust gases, solid and liquid residues, effluents and products. The main sources of PCDDs and PCDFs are classified into four categories, namely incineration, combustion, and industrial processes. They also can be formed by natural processes such as volcanic eruptions and forest fires.
Of all of the isomers, one, 2,3,7,8-tetrachloro-p-dibenzo-dioxin (2,3,7,8 TCDD), is considered the most toxic. The U.S. Environmental Protection Agency (US EPA) has said that it is likely to be a cancer-causing substance to humans. In addition, people exposed to dioxins and furans may experience changes in hormone levels. High doses of dioxin have caused a skin disease called chloracne. PCDDs and PCDFs are not made for any purpose but they are created as a byproduct when products like herbicides are manufactured. They are also created in the pulp and paper industry, from a process that bleaches the wood pulp. In addition, they also can be produced when certain products of organic chlorine are burned.
There are several sources of exposure to PCDDs and PCDFs. If people work near a municipal solid waste incinerator, copper smelter, cement kiln or coal-fired power plant, they can be exposed to dioxins and furans. Individuals who burn their household waste or burn wood can be exposed as well. Even forest fires can create small amounts of PCDDs and PCDFs in the air.
Most PCDDs and PCDFs can enter the body by breathing contaminated air, drinking contaminated water or eating contaminated food. About 90 percent of exposure to PCDDs and PCDFs is from eating food grown in contaminated soil or eating animals that consume the contaminated feed. Over time, dioxins and furans can build up in the fatty tissues of animals. The U.S. EPA has set a limit of 0.00003 micrograms of 2,3,7,8-TCDD per liter of drinking water (ug/L). The Food and Drug Administration recommends not eating fish and shellfish with more than 50 parts per trillion of 2,3,7,8-TCDD.
Based on the evidence presented, it’s possible the control burn process resulted in elevated vapor exposure to VCM for emergency responders venting the tank car. Modeling of the potential VCM exposure to workers and the public was not performed to evaluate the ambient airborne exposure to the residents. Emergency responders most likely were exposed to significantly elevated airborne VCM concentrations. Reliance on information from the 2020 National Fire Protection Association Emergency Guidance document without using pre-planning based on the ALOHA or CAMEO models did not provide the Unified Command System with the necessary health and safety information other than how to prevent a BLEVE. Could the VCM burn produce PCDDs and PCDFs and release the contaminants into the ambient environment? No peer-reviewed published research exists to answer this question. Without conducting a scientific study of the soil and groundwater at the railroad derailment site, the answer remains unclear but the potential certainly exists.