
Sources and Measurement of Toxic Chemicals
The PID is one of the most sensitive detectors available for many toxic compounds with detection limits in the low or sub-ppb range.
As we learn more about chemicals and their interaction with the human body, we find that some chemicals are even more toxic than originally thought. An example of this occurred in 1973 when the permissible exposure level (PEL) for vinyl chloride (VC) was reduced from 500 ppm to 50 ppm, then to 1 ppm, over a period of a year because of its link to cancer. We developed the first photoionization detector (PID) in 1973 and applied it to measuring VC in 1974. It became the only portable analyzer that could measure VC at the 1 ppm level and protect the workers.
NIOSH has a list of nearly 400 chemicals that are termed Immediately Dangerous to Life or Health (IDLH). In 1990, EPA published a list of 189 air toxics that were hazardous air pollutants known to cause or suspected of causing cancer. We will discuss a number of situations involving toxic chemicals and various government agencies. We will also describe some instrumentation that could be used for monitoring to prevent or minimize exposures to toxics.
What government agencies are involved with measuring and/or controlling toxics? These include: OSHA, EPA, Homeland Security (DHS), USDA, FDA, and DEA. The latter two agencies will not be discussed here.
A toxic substance can be defined as any chemical that, through its chemical action on the human body, can cause death, temporary incapacitation, or permanent harm.
Where can toxics be encountered?
- Hazmat: Accidental chemical spills—truck or railcar carrying chemicals; gasoline—EPA, OSHA
- Workplace during manufacturing—OSHA
- Terrorism: subway, stadium, public gathering—DHS
- Air toxics: exposure in an area surrounding a manufacturing or chemical plant or a mobile source—EPA Clean Air Act
What can be used to measure these toxic compounds? A portable PID or gas chromatograph (GC)/PID that will be discussed in this paper.
The equipment should be lightweight, battery operated, rugged, sensitive (ppb) or even sub ppb (with a concentrator), and, most of all, easy to use by associate safety professionals, certified industrial hygienists, first responders, and/or technicians.
The photoionization detector (PID) with a GC has low ppb sensitivity via direct injection, requires no support gases, and can be used with a concentrator to measure ppt levels of toxic compounds. The toxics detected can include: chemical agents, benzene, vinyl chloride, acrolein, hydrazine, chlorinated hydrocarbons, etc.
Toxics Areas
OSHA
Some chemical intermediates can be more toxic than the final chemical products. An example of this is methyl isocyanate (MIC), which is used to make carbamate pesticides such as carbaryl. This well-known chemical was involved in an accidental release in 1984 in Bhopal, India, that resulted in the death of nearly 4,000 and injury to more than 500,000 people. Most recently, in March 2016, a poisoning of 102 persons in Batu Gajah, Malaysia, via carbamate contamination in food resulted in at least one death. The IDLH for MIC is 20 ppm, and the PEL is 20 ppb. The PEL of carbaryl is 605 ppb. Although still low, it has a PEL that is 30 times higher than the intermediate product.
Reaction vessels are used in carrying out a wide variety of chemical reactions, including oxidation, polymerization, esterification, hydrolysis, hydration, and hydrogenation. There have been situations where a reaction vessel was checked with a four-gas meter (combustibles, oxygen, hydrogen sulfide, carbon monoxide)—everything was normal, then a worker went into the tank without a respirator and died. The cause: A toxic chemical was in the kettle that was above the IDLH (ppm value). The combustible gas sensor was not sensitive enough to detect this compound. An alternative to the conventional four-gas meter is a four-gas meter that can detect low or sub ppm levels of most organic compounds at ppb levels, in addition to O2 and CO. The American Industrial Hygiene Association’s Real-Time Detection Systems Committee is working on a four- gas PID method that could be used to prevent this type of exposure to toxics.
IDLH
The current NIOSH definition for an Immediately Dangerous to Life or Health (IDLH) condition is one that poses a threat of exposure to airborne contaminants when the exposure is likely to cause death or immediate or delayed permanent adverse health effects or prevent escape from such an environment [NIOSH 2004]. The gas chromatograph/PID responds to most organic compounds (not methane) and certain inorganic compounds, such as: hydrogen sulfide, hydrogen selenide, chlorine, bromine, nitrogen dioxide, ozone, and phosphine.
Fumigants can be dangerous because some are very toxic and a number of accidental deaths have occurred. For example, three fumigants (a soil fumigant, Chloropicrin; a grain fumigant, phosphine; and sulfuryl fluoride, a structural fumigant) have IDLHs of 5 ppm, 50 ppm, and 200 ppm, respectively.
Hazmat (EPA/OSHA)
A spill from an overturned railcar or tanker could be from small amount to 5,500-11,000 gallons of liquid cargo. If this material is volatile, 1 cc of liquid (not pressurized) would be converted into 1L of vapor, and one gallon of liquid contains 3,800 cc (3.8 million liters of gas) X volume spilled. In many cases, the chemical spilled has been liquid chlorine or ammonia. Chlorine has been used in the Middle East as a chemical agent and is quite toxic (the IDLH for chlorine is 10 ppm). During the 1980s and 1990s, the HNU Systems PID Analyzer was one of the most useful tools to protect workers from VOCs at Superfund sites. At the Chemistry of Industrial Hygiene reception at the American Industrial Hygiene Conference and Exposition in Portland, Ore., in 2011, a Superfund contractor for the Environmental Protection Agency said to Jack Driscoll, who first commercialized photoionization in 1973, "I want to thank you and your PID for saving thousands of lives at Superfund sites."
The challenge at a hazmat site is how to protect the first responders; evacuate the people; contain the leak; minimize the contaminated area, including surrounding areas of concern such as water and soil; and clean up the spill. This is a complex task. A portable PID Analyzer and a portable GC with a PID are very useful tools to provide worker protection and protection of people in the immediate area, as well as to determine the extent of contamination in the soil and in any nearby water sources.
Hazmat training is a requirement (OSHA 1910.1920) for the operators at a spill site. For large releases, a command center would be set up and a mobile van with portable instrumentation such as four-gas meters, PIDs, flame ionization detectors (FIDs), and portable GCs would be employed.
Homeland security
Chemicals such as chlorine and pesticides have been used over the past decade primarily in Afghanistan, Iraq, and Syria to kill and injure large groups of people (soldiers, children, and civilians).
Unlike chlorine, chemical agents are more difficult to prepare, and many of the chemicals used in their preparation are regulated by the government. However, on March 20, 1995, Sarin was released in a subway in Tokyo, Japan, by Aum Shinrikyo, a Japanese terrorist group. Twelve people were killed and up to 5,000 people were treated for the effects of the Sarin. Visiting the Japanese EPA the next days via a subway station in the basement of the Japanese EPA office building, it was quite peculiar that no one at the meeting acknowledged the event of the previous day.
The University of Helsinki has evaluated the response of chemical agents with our portable GC/PID. The results are shown in Figure 1. The PID has good sensitivity (sub-nanogram detection) for many chemical agents.
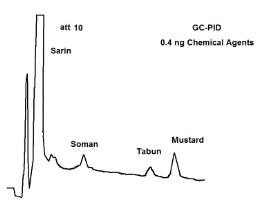 Figure 1
Figure 1
Terrorists will try to strike at targets where there are large numbers of people, so an area monitor such as a GC/PID would be useful to detect any unusual chemicals in the area. Chemical and biological agents could be used by terrorists to target our embassies and/or military bases in foreign countries. Homeland Security also regulates the security of high-risk chemical facilities.
Clean Air Act
The Clean Air Act 1970 focused on reducing SOx, NOx, and particulates in the ambient air. The Clean Air Act of 1990 continued with additional emphasis on new power plants but expanded its focus to glass plants, cement plants, and reducing mercury from coal-fired power plants. When Congress passed the Clean Air Act of 1990, it contained 189 Hazardous Air Pollutants (HAPS) to be regulated, and fence line monitoring of many of these chemicals was suggested.
EPA has continued to work on mobile sources. One of the priorities was the regulation of benzene in gasoline. Studies were initiated in New York City, where a significant drop in benzene in ambient air was detected after the benzene concentration in gasoline was reduced. At the American Chemical Society Fall meeting in Boston 2015, we presented research on some ambient air measurements of aromatics in Sandwich, Mass. We found 54 ppt of benzene in ambient air in Sandwich (Figure 2), while previous measurements of benzene reported by us in Newton during the 1990s were in the range of 5-10 ppb. It appears that benzene in the ambient air has been reduced significantly.
The chromatogram below indicates a detection limit of less than 1 ppb. It would be useful to have a battery-operated concentrator so that ppt levels of benzene could be detected in the field. A PID/GC may need more sensitivity, so we have developed a new concentrator that is battery operated. The data is shown below.
In October 2015, EPA started to focus on proposed fence line monitoring of benzene at refineries as part of its HAPs program. EPA proposed an alarm level for monitoring benzene at the fence line of 2.8 ppb.
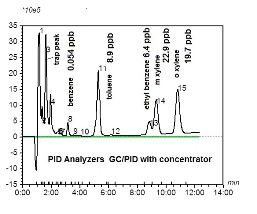 Figure 2
Figure 2
A GC/PID with the proper chromatography can be configured to be specific for benzene (no interferences from other hydrocarbons) in a refinery fence line area. The GC would have to detect 1/10 of the EPA alarm level, or about 250 ppb. This would require a concentrator that could be ramped up and cooled down quickly to concentrate the benzene. The total analysis could be done in less than two minutes.
Conclusions
The PID is one of the most sensitive detectors available for many toxic compounds with detection limits in the low or sub-ppb range. Portable GCs with a PID can be equipped with a battery-operated concentrator to achieve detection of ppt levels of toxics or HAPs in the field.
A normal four-gas detector that has only a combustible gas sensor should not be used for areas where toxic gases are present. It should be replaced with a four-gas PID that would respond to the toxic gases present. This would maximize the protection of the worker. This four-gas PID Analyzer can be used to check manufacturing processes and any tanks used in production processes or pilot plants.
A combination of PIDs and a GC/PID with a methyl silicone capillary column would be most useful tool for the first responders in an area where there was a railcar or tanker spill. A headspace method could be rapidly employed to check VOC contamination in the soil or water.
For Homeland Security, a GC with a PID/FID in series would be most useful since the PID/FID ratio can be used to determine the type of hydrocarbon that is eluted, because this ratio varies with the structure (alkane, alkene and aromatic for the PID, but the response remains the same for the FID). A concentrator would be essential because low levels can be detected before there is a serious problem. The battery operation of both the GC and concentrator is ideal because the equipment can be easily and quickly moved to a different area. The GC/PID will also detect chemical agents.
For Clean Air Act monitoring, the GC/PID will respond to many of the 189 HAPs in the program, so it would be ideal for fence line monitoring surveys. A compact GC/PID Analyzer that would continuously monitor benzene at fence lines is available to meet the monitoring deadline, which is less than two years away.
This article was developed from research presented at the American Chemical Society Spring 2016 meeting in San Diego, Calif., in the Advances in Analytical Separations symposium on the Analytical Chemistry Division track.
This article originally appeared in the May 2016 issue of Occupational Health & Safety.