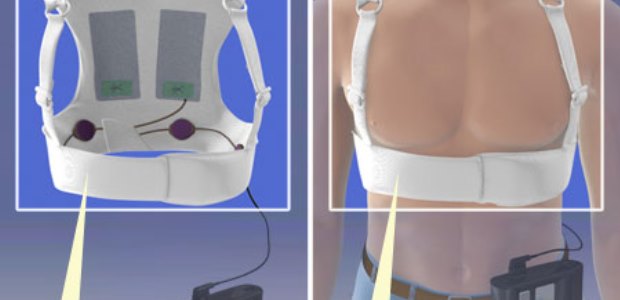
LifeVest Defibrillator Approved for Pediatric Use
"Doctors now have important information that may help them safely prescribe this life-saving device to young patients who may benefit from the device," said Dr. Vasum Peiris, M.D., MPH, chief medical officer of Pediatrics and Special Populations in FDA's Center for Devices and Radiological Health.
The U.S. Food and Drug Administration on Dec. 17 approved a new indication for Zoll's LifeVest wearable cardioverter defibrillator, approving it for use by some children who are at risk for sudden cardiac arrest but are not candidates for animplantable defibrillator due to certain medical conditions or lack of parental consent. Specifically, the approval allows its use by children who weigh at least 41 pounds and have a chest size of 26 inches or more, about the size of an average 8 year old, the agency announced.
"The pediatric medical community is often forced to use adult devices off-label without appropriate labeling or instructions for use in pediatric patients," said Dr. Vasum Peiris, M.D., MPH, chief medical officer of Pediatrics and Special Populations in FDA's Center for Devices and Radiological Health. "Doctors now have important information that may help them safely prescribe this life-saving device to young patients who may benefit from the device."
According to FDA, many AEDs are approved for use on children, but LifeVest is the only one worn by the patient that monitors the heart continuously for arrhythmias. The device responds automatically if it senses the need to deliver a shock.
The LifeVest was initially approved in 2001 for patients 18 and older, and later models were approved for adults in 2002, 2006 and 2009. It is manufactured by Zoll in Pittsburgh, Pa. Zoll Medical Corporation is based in Chelmsford, Mass.
FDA said the pediatric approval was based on published studies and a company registry containing clinical information from 248 patients, ages 3 to 17, at risk for sudden cardiac arrest. "No additional safety concerns were identified, and four patients who experienced sudden cardiac arrest received a shock that successfully restored a life-sustaining heartbeat," according to its announcement.