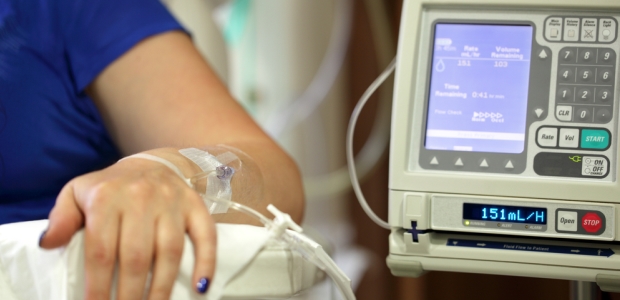
Joint Commission Report Explains New Clinical Alarm Goal
It requires accredited hospitals and critical access hospitals to improve the safety of their clinical alarm systems and is being implemented in two phases.
The Joint Commission on Dec. 11 released an "R3 Report" -- the letters signify Requirement, Rationale, and Reference -- for the new National Patient Safety Goal that requires accredited hospitals and critical access hospitals to improve the safety of their clinical alarm systems. The brief report gives accredited health care organizations and health care professionals information about the development of the goal.
Phase one goes into effect Jan. 1, 2014, and raises awareness of the potential risks associated with clinical alarms from machines such as cardiac monitors, IV machines, and ventilators. The second phase takes effect Jan. 1, 2016, and introduces requirements to mitigate the risks. Generally, this goal does not cover items such as nurse call systems, alerts from computerized provider order entry, or other IT systems, according to the commission's news release about the report.
The report explains why the goal is needed, activities that led up to the development of the goal (including a summit convened by the Joint Commission, the Association for the Advancement of Medical Instrumentation, ECRI Institute, the American College of Clinical Engineering, and the Food and Drug Administration), and steps health care leaders at affected institutions should take to meet the requirements.