
Twenty-one percent of the 262 people who sustained non-fatal injuries when the ammonium nitrate facility exploded suffered a TBI, according to a report released June 24 by public health officials in Texas.
The organization releases a set of recommendations to protect children from lead poisoning in developing countries.
The agency released a robust FAQ section regarding the recent respirable dust rule's medical examination requirements.
More than 800 new cases have been reported during the past two weeks, the California Department of Public Health’s director reported June 13.
At Safety 2014, attendees learned what the new era of big data means for safety professionals.
The agency cites the company for violating the Federal Railroad Safety Act’s anti-discrimination provisions.
FDA's final order requires a warning label on sunlamp products and UV lamps that states they should not be used by anyone under 18.
With 288 cases reported to it as of May 23, the agency is urging people without them to be vaccinated as 2014’s summer travel season nears.
The agency made the announcement in the wake of two mining stakeholder safety summits and 20 mining deaths since October 2013.
The agency has approved the first-ever implantable wireless device with remote monitoring to measure pulmonary artery pressure and heart rate in patients with heart failure.
Doctors will try to save seriously injured patients -– dying patients, actually -– by using the procedure.
The association's Health Care Section is sponsoring the June 11 panel.
The resolution notes the importance of implementing measures to protect vulnerable groups and says there is a need to improve screening.
The number reported this year through April, 1,711, more than tripled the number during the same period in 2013.
The agency has announced it will be requiring a lower starting dose for the sleep drug.
May 13’s decision took place at the fifth meeting of the International Health Regulations Emergency Committee concerning Middle East respiratory syndrome coronavirus. WHO's statement says the committee members did agree their concern about the situation has significantly increased, given the recent spike in cases.
The agency approves Zontivity to reduce the risk of heart attack, stroke and cardiovascular death in high risk patients.
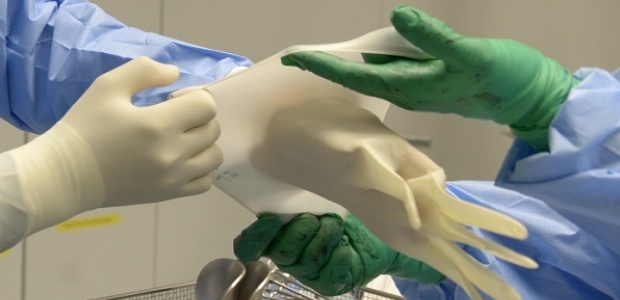
ASTM has formed a task group to develop a specification standard for them, Selcen Kilinc-Balci, a physical scientist for NIOSH's National Personal Protective Technology Laboratory, reported on the agency’s Science Blog.

Dr. Margaret A. Hamburg writes that the states "have an important role to play in addressing a critical driver of opioid abuse -- inappropriate prescribing practices. However, we can't just focus on one drug, Zohydro, alone."
As many as 75 percent of the recent cases are secondary infections -- meaning individuals who became infected through contact with another person. Most of these are health care workers who became infected at work.