
Using Half-Facepiece Respirators for H1N1
- By Scott Larson
- Nov 03, 2009

Photo courtesy of NIOSH via Flickr.
With the recent outbreak of H1N1 virus around the globe, there has been increased interest in using respirators to help protect people at work, at home, and while out in public. While most people have seen or used respirators, few people truly appreciate and understand how these apparently simple devices actually work and what is required to use them properly in order to receive the expected protection that they can offer. Improper use can lead to user being overexposed, possible OSHA citations, and, most importantly, users potentially suffering needlessly from an occupational disease. Th is article will focus on understanding one of the most common types of negative pressure respirators being used: a half facepiece with a particle filter.
The purpose of a respirator is to help reduce the wearer's exposure to airborne contaminants and to bring that exposure to within an "acceptable" level as determined by regulatory agencies and/or health organizations. However, for some contaminants, such as biological aerosols, no acceptable exposure levels (i.e., the amount you can inhale without adverse health effects) have been established. Therefore, while respirators can help reduce exposures to biological aerosols, they cannot eliminate the risk of contracting infection, illness, or disease. No respirator will provide the wearer with a zero exposure level.
The use of respirators in occupational settings, including health care, is regulated by OSHA. Its Respiratory Protection Standard, 29 CFR 1910.134, governs the selection and use of respirators for occupational applications.
Half-Facepiece Respirators
A respirator is a device designed to help provide the wearer with respiratory protection against inhalation of a hazardous atmosphere. They are tested and certified by the National Institute for Occupational Safety and Health (NIOSH). NIOSH tests particulate respirators under "worst case" conditions to help ensure adequate performance in the workplace. The test protocol includes high flow rate, particles in the most penetrating size range, aerosols that may degrade filter material, etc. Filtering facepiece respirators that are approved under these tests must have "NIOSH" and the filter classification printed on them. The overall level of protection offered by a NIOSH-certified respirator is described by the Assigned Protection Factor, or APF, which is the minimum anticipated protection provided by a properly functioning respirator or class of respirators to a given percentage of properly fitted and trained users. The APF takes into account all expected sources of facepiece penetration, such as face seal penetration, filter penetration, and valve leakage. A properly fitted and used half-facepiece, NIOSHapproved respirator has an APF of 10, which means it will reduce the exposure by a factor of 10.
A negative-pressure half-facepiece respirator is one that seals tightly to the face and fits over the nose and beneath the chin, covering your nose and mouth. When a user inhales the air pressure inside, the facepiece is negative with respect to the ambient air pressure outside the respirator. A person's lungs are the mechanism that draws air through the filter. The two common types of negative-pressure halffacepiece respirators are elastomeric (reusable) and filtering facepiece. An elastomeric respirator is commonly made from synthetic rubber or silicone material, and filters are attached to the facepiece. A filtering facepiece respirator (FFR) is where the filter composes the entire respirator.
Surgical Masks
Because of certain similarities in appearance, surgical masks are sometimes confused with filtering facepiece respirators. Although both cover a user's nose and mouth, they do not perform the same function. A surgical mask is an infection control device designed to help prevent the spread of infection from the wearer's exhaled breath to potentially susceptible persons. Surgical masks do not have either adequate filtering or fitting attributes to provide respiratory protection for the wearer. They are designed to help prevent contamination of the work environment or sterile field from large particles generated by the wearer (e.g., spit, mucous).
Surgical masks also may be used to help reduce the risk of splashes or sprays of blood, body fluids, secretions, and excretions from reaching the wearer's mouth and nose. However, because surgical masks are not designed nor tested the same as respirators, any "filtration efficiency" claims for them cannot be directly compared to those for a respirator. Most surgical masks do not seal tightly to the face, which allows air to pass around the edge of the mask. Surgical masks do not have APFs and should not be used to help reduce exposures to particles in the air.
In a few cases, a NIOSH-approved respirator may also have the attributes of a surgical mask. These are typically referred to as a "surgical respirator." Th is means it can help filter large droplets expelled by the wearer but has also been shown to have efficacy at filtering smaller particles and is designed to fit tightly to the face. Because of its additional use as a respirator, this type of surgical mask also must be fit tested. If the wearer needs a combination surgical mask and a particulate respirator, he should use a product that is both cleared by FDA as a surgical mask and approved by NIOSH as a particulate respirator.
Importance of a Properly Fitting Respirator
Fit is very important. If a respirator does not seal tightly to the face, airborne hazards can penetrate or enter underneath the facepiece seal and into the wearer's respiratory system. It is very important to always follow the donning instructions and do a user seal check each time the respirator is put on and before entering a contaminated environment. A good fit can be obtained only if the wearer's face is clean shaven in the area where the respirator seals against the face. Beards, long mustaches, and stubble may cause leaks into the respirator. Hair, jewelry, and clothing should not be between the face and the respirator.
Many medical facemasks, not approved as respirators, do not seal tightly to the face, which allows airborne hazards to enter the respiratory system. Even those medical facemasks that appear to seal tightly to the face have not been designed to protect the wearer from airborne hazards. Therefore, they should not be considered an equivalent substitute for government-approved respirators.
In the United States, workers required to wear respirators are mandated by OSHA to be fit tested prior to use and must conduct user seal checks before each use of the respirator in a contaminated area. The purpose of fit testing is to help train the worker in proper fit techniques and determine the respirator style and size that will properly fit the wearer. OSHA requires this to be done prior to initial use, whenever a different make or size respirator is used, and annually thereafter. There are two types of fit tests: qualitative and quantitative. In qualitative fit testing, taste, smell, or irritation is used as a sign of an improperly fitting respirator. Quantitative fit testing uses a direct reading instrument to evaluate the fit of the respirator, such as one that counts the number of particles inside and outside the respirator.
Particle Filtration
Particulate filters and respirators are used for filtering dusts, fumes, mists, and bioaerosols. Filtration efficiency is one of the tests used for certification. These tests are designed to be very stringent, or "worst case." Filtering facepiece respirators approved with N95 level filters are often referred to as "N95 respirators" or "N95s." Of the nine NIOSH filter classifications, those rated N95 are the most widely used.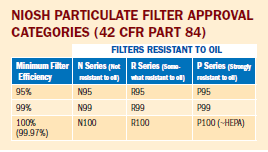
The difference between a half-facepiece respirator with an N95 level filter and a one with a P100 level filter is that the P100-level filter provides higher filtration efficiency under laboratory conditions than the N95 filter. However, the overall level of protection afforded by either respirator outside the laboratory (i.e., APF) will not be changed. It should be noted that penetration of particles through the filter is only one of the possible sources of exposure to contaminants. Other potential sources, such as face seal leakage, improper maintenance, or not wearing the respirator when necessary, may contribute more to exposure than filter penetration. Each of these conditions must be addressed and controlled.
When to Change Filters
Manufacturers typically recommend all particle filters be replaced when they become dirty, damaged, or the wearer notices an increased breathing resistance. In addition, if R series filters are used in oily atmospheres, they are required to be disposed of at the end of each shift , regardless of condition. When P series filters are used in oily atmospheres, the manufacturer must provide guidance when to change. Many manufacturers, including 3M, recommend they be disposed of 30 days after first being put into service or after 40 hours of use, whichever comes first. For disposal procedures after exposures to infectious aerosols, the user should review the respirator user instructions and consult with his or her infection control officer, industrial hygienist, or supervisor.
To use a respirator correctly, the worker must be properly trained, and ensuring workers are properly trained is the employer's responsibility. Just as important, the respirator training provider must be trained and knowledgeable. While a respirator may appear to be fairly simple item, proper use and understanding of its limitations can be complex. The trainer must have a complete knowledge of the specific respirator to be used — how it works, restrictions, warning indicators of improper use, as well as an understanding of the regulations regarding respirator use in general. Review of the information in the manufacturer's user instructions should be part of any training program.
Summary
A respirator is a device designed to help provide the wearer with respiratory protection against inhalation of airborne contaminants. Increasing the filtration level of a particle respirator does not increase the respirator's ability to reduce a user's exposure to contaminants. The APF of a respirator, which is affected by the respirator style, determines the potential for exposure reduction. Surgical masks that are not approved as filtering facepiece half-mask respirators do not have an APF and should not be used for reducing workers' exposures to particles in the air.
This article originally appeared in the November 2009 issue of Occupational Health & Safety.