
Page 4 of 6
An Overview of SAMHSA's New Oral Fluid Testing Guidelines
Workplace drug testing may never be the same. After 30 years of only permitting lab-based urine testing, the Substance Abuse and Mental Health Services Administration (SAMHSA) published its final Mandatory Guidelines for Federal Workplace Drug Testing Programs using Oral Fluid on Oct. 25, 2019 in the Federal Register. The new regulations only apply to federal workplaces, at this time, but the impact is sure to reach beyond the initial scope of the regulations.
Consider this: SAMHSA’s mandatory guidelines have served as the blueprint for many state drug testing laws, corporate drug testing policies, and the legal argument for how to drug test the right way since the original guidelines were published in 1988. Even though alternative testing methods—including hair testing, instant-result devices, and, yes, lab-based oral fluid testing—have been around for many years, they have not put a significant dent in the market share of lab-based urine testing in the workplace. According to SAMHSA, that’s about to change.
In the original Notice of Proposed Rulemaking (NPRM) for oral fluid testing, as well as in the final regulatory language made public in October 2019, SAMHSA projected that about seven percent of federal drug tests would transition from urine to oral fluid in the first year. Furthermore, the agency predicted that in four years, 25 to 30 percent of all federal workplace drug tests would be conducted utilizing oral fluid. But if that was all, the overall impact of the Oral Fluid Mandatory Guidelines (OFMG) on the drug testing industry would be minimal, at best.
However, SAMHSA also predicted the same transition rate for the drug tests mandated by the U.S. Department of Transportation (DOT) (approximately 6 million annually) and the Nuclear Regulatory Commission (NRC) (about 155,000 per year). Projecting a 25 to 30 percent transition rate for DOT and NRC represents a significant impact on the industry.
But what about the approximately 40 million workplace drug tests not regulated by the federal government? If we realize the same transition rate, which is well within the realm of possibility, we suddenly have a fundamentally transformed drug testing industry. This is just in time as state after state not only legalizes marijuana but also looks to place restrictions on employers’ rights to test for THC and/or hold applicants and employees accountable when they test positive.
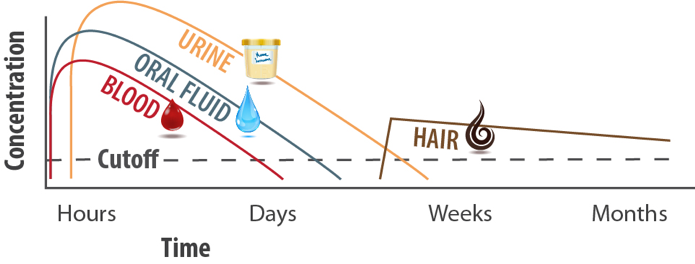
The chief argument behind most of these legal actions is the inability to claim someone is “impaired” solely based on the result from a drug test with a window of detection of days or weeks. Oral fluid, with its tighter window of detection (think hours rather than days), gives an almost immediate result that would show a person’s peak levels of intoxication at the time of the test on the job. It’s a faster approach for more timely results.
But let’s not get ahead of ourselves. SAMHSA began teasing providers and employers years before the marijuana legalization movement really took off. The dedicated staff and experts at SAMHSA and the Drug Testing Advisory Board, as well as the all-star teams at DOT and NRC, deserve a tremendous amount of credit for patiently resolving every concern from within and outside the government and persevering long enough to finally issue regulations for a completely different drug testing method. While these new guidelines are not a direct response to what’s happening with marijuana laws across the country, they do align well with the controversial trend toward full legalization.
So, if these new regulations are not the federal government’s response to legalization, then why did SAMHSA spend years on this project? Why after 30 years did an agency not known for change or flexibility (no offense intended), suddenly turn an entire industry on its head? There were actually many good reasons, but three seem to stand out the most: combatting adulteration, cutting the time and costs of a typical drug test, and the science of the technology behind lab-based oral fluid testing.
Regarding drug test cheating, SAMHSA states:
All unobserved specimen collections are at risk for substitution and adulteration. Studies conducted by the drug testing industry indicate that 0.05 to 3 percent of urine specimens collected for drug use detection are determined to be substituted or adulterated. Oral fluid collections will occur under observation, which should substantially lessen the risks of specimen substitution and adulteration that has been associated with urine specimen collections, most of which are unobserved.
On the subject of cutting time and costs, SAMHSA offered the following: “Oral fluid collection can require less time than urine collection, reducing employee time away from the workplace and, therefore, reducing costs to the…employer.”
In the OFMG, SAMHSA also emphasized that oral fluid collections do not require a facility that provides visual privacy during the collection and that the agency anticipates that “many oral fluid collections will occur at or near the workplace, and not at a dedicated collection site, thereby reducing the amount of time away from the workplace.”
Employers will have the option to collect a urine specimen when a donor is unable to provide an oral fluid specimen (and vice versa), which will “reduce both the need to reschedule a collection and the need for the MRO to arrange a medical evaluation of a donor’s inability to provide a specimen.”
Perhaps, the greatest time and cost savings cited by SAMHSA was the reduction in the overall time needed to administer an oral fluid test.
Administrative data for urine collections indicates it takes, on average, about four hours from the start of the notification of the drug test to the actual time a donor reports back to the worksite… The Department estimates the time savings to be more than 2 hours. Using OPM’s estimate for the average annual salary of Federal employees converted to an hourly wage, the savings generated for the Federal Government would be roughly $400,000 to $1.2 million a year, or $38 to $114 per test.
In the Federal Register announcement, SAMHSA stated:
The scientific basis for the use of oral fluid as an alternative specimen for drug testing has now been broadly established and the advances in the use of oral fluid in detecting drugs have made it possible for this alternative specimen to be used in federal programs with the same level of confidence that has been applied to the use of urine.
SAMHSA added:
The OFMG provide the same scientific and forensic supportability of drug test results as the Mandatory Guidelines for Federal Workplace Drug Testing Programs using Urine (UrMG).
That’s quite an acknowledgement. And phrases like “scientific basis… has been broadly established” and “the same scientific and forensic supportability” as urine testing will likely have a powerful impact on employers, mandated or not, who are considering labbased oral fluid testing. It will force many service providers from a passive marketing position of simply making oral fluid testing available to clients to more aggressive and outward efforts to not only offer this alternative testing method but to differentiate themselves from their competition.
For the typical buyer, as well as many sellers of drug testing, it’s more about authorization than it is about science: “If it’s good enough for the government, it must be good enough for me.” That may be a gross oversimplification of an otherwise complex issue, but it applies, generally. Some people must now, at the very least, become knowledgeable about lab-based oral fluid testing, while others—such as lab professionals, collectors, certain employees at companies that conduct drug testing, and medical review officers (MROs)—must become experts, at least in the specific duties they will be responsible for in an OFMG-compliant testing program.
SAMHSA is allowing a 12- to 18-month implementation period to give employers and service providers ample time to learn the regulations, get trained in how to fulfill their responsibilities per the guidelines, and in some cases (such as with laboratories), become certified. Fortunately, the OFMG are written in the same concise, easy-to-understand style as the mandatory guidelines for urine testing, which is a good thing because there’s a lot to know going forward. Following are just a few highlights of the OFMG.
What Must Collectors Know?
Collectors are significantly affected by the OFMG. First, with oral fluid collections, employees, in addition to professional technicians, can conduct the collection. There are two key parts to collector training: the OFMG and the specific collection device being used. The OFMG define a collector as someone “who has been trained to collect oral fluid specimens in accordance with these Guidelines and the manufacturer’s procedures for the collection device.”
Second, collections can take place at the workplace in addition to an approved, offsite facility. The site being used may be a permanent or a temporary facility if the site meets all the requirements of an approved collection site. SAMHSA anticipates that many employers will choose to collect oral fluid samples at the work site in order to save time and boost productivity. Collectors, including employees who conduct collections, will be responsible for ensuring the portion of a worksite being used for collections qualifies, according to the OFMG. Citing Subpart E of the guidelines, collection site requirements include:
- Provisions to ensure donor privacy during the collection
- A suitable and clean surface area not accessible to the donor for handling specimens and completing the required paperwork
- A secure temporary storage area to maintain specimens until the specimen is transferred to an HHS-certified laboratory
- A restricted access area where only authorized personnel may be present during the collection
- A restricted access area for the storage of collection supplies
- The ability to store records securely
The federal custody and control form (CCF) must be used with each collection in much the same way it is used currently for urine specimen collections. With few exceptions, “The OMB-approved Federal CCF must be used to document custody and control of each specimen at the collection site.”
Collectors may only use a single-use collection device designed specifically for oral fluid collections that has been cleared by the Food & Drug Administration (FDA). The device must “not substantially affect the composition of drugs and/or drug metabolites in the oral fluid specimen.”
Among the requirements for FDA clearance, a device must have a built-in volume indicator and be capable of collecting a least 1 mL of “undiluted (neat) oral fluid.”
Split specimen collections are required. Serial or simultaneous collections using two collection devices constitute a split oral fluid collection. According to the OFMG:
The collector collects at least 1 mL of undiluted (neat) oral fluid in a collection device designated as ‘A’ (primary) and at least 1 mL of undiluted (neat) oral fluid in a collection device designated as ‘B’ (split) either simultaneously or serially (i.e., using two devices or using one device and subdividing the specimen).
What Must Laboratories Know?
The easy answer to this question is “a lot.” While not all laboratories will choose to offer OFMG-compliant oral fluid drug testing, those that do must become certified by successfully going through a very similar and equally rigorous process required of labs that offer urine drug testing. In other words, the certification process is not a proverbial “walk in the park.” And that’s good news for employers and drug testing providers who have come to trust and rely on the SAMHSA-lab certification as a quasi “good housekeeping” seal of approval.
Laboratories certified to conduct oral fluid tests must test for marijuana and cocaine, and they are authorized to test for opioids, amphetamines, and phencyclidine. On a case-by-case basis, when certain requirements are met (typically involving reasonable suspicion or post-accident testing), a specimen may be tested for any drugs listed in Schedule I or II of the Controlled Substances Act (CSA).
Also, laboratories will use SAMHSA-approved cut-off levels designed to show the presence of drugs in a manner similar to urine cut-off levels. The cut-off level for THC was a topic of much discussion prior to the issuance of the final guidelines. According to the OFMG, the initial screen cut-off level for marijuana is 4 ng/mL and 2 ng/mL for confirmation tests. Remember, oral fluid tests with labs detect the parent drug (the drug itself), whereas urine labs typically detect a metabolite of the drug. A metabolite is a substance formed when the body breaks down food, drugs or chemicals, or its own tissue as part of its metabolism.2
Because of this, urine tests have the ability to detect “recent use” with oral fluids.
It is probably a safe prediction to say that the day is not far distant when using a SAMHSA-certified laboratory to conduct oral fluid testing will be considered by many to be the preferred method, in much the same way that using a certified lab to test for urine has been the preferred way for three decades.
What Must MROs Know?
When reading the OFMG, it is clear that protecting the integrity of the testing process and ensuring the accuracy of reported results were of paramount importance to SAMHSA during the development of the guidelines. As is the case with urine drug testing, MROs play a critical role in the OFMG.
In some ways, not a lot has changed for MROs except for the fact that they will be verifying results from a completely different drug testing method. So, there is much in the way of added responsibilities. As such, MROs must learn the details of the OFMG and everything related to reviewing and verifying oral fluid test results.
For employers and providers who may still be on the fence regarding lab-based oral fluid drug testing, consider the following regarding the role of MROs:
The OFMG require that MROs: (1) Review the information on the MRO copy of the Federal CCF that was received from the collector and the report received from the HHS-certified laboratory or HHS-certified IITF; (2) Interview the donor when required; (3) Make a determination regarding the test result; and (4) Report the verified result to the federal agency.
Getting Ready
The Oral Fluid Mandatory Guidelines will initially only apply to federal workplaces. DOT has stated its intention to have its own oral fluid testing regulations in place by the time SAMHSA completes its 12- to 18-month implementation period.
The OFMG are an official endorsement of lab-based oral fluid testing by the federal government, and the guidelines provide a new “gold” standard for how to best utilize the technology. Many employers will consider this a green light to implement oral fluid testing either in place of, or in combination with, urine drug testing (lab-based oral fluid testing has historically been permitted in 47 states).
For the professionals who ensure the integrity of each drug test, such as collectors, labs, and MROs, the OFMG will become the “gold” standard for oral fluid drug testing. For employers, the OFMG will provide a level of comfort when utilizing lab-based oral fluid drug testing. For both groups, service providers and end users of their services, now is the time to prepare.
REFERNCES
1 https://www.federalregister.gov/documents/2019/10/25/2019-22684/ mandatory-guidelines-for-federal-workplace-drug-testing-programs-oralfluid
2 https://www.cancer.gov/publications/dictionaries/cancer-terms/def/ metabolite
This article originally appeared in the March 2020 issue of Occupational Health & Safety.