CDC Calls Microneedle Patch Possible 'Game Changer'
Designed to be administered by minimally trained workers, it would simplify storage, distribution, and disposal compared with conventional vaccines.
CDC sees a lot of potential in a microneedle patch it is developing with the Georgia Institute of Technology, calling it a possible game changer that will make it easier to vaccinate people against measles and other vaccine-preventable diseases. The patch is designed to be administered by minimally trained workers and to simplify storage, distribution, and disposal compared with conventional vaccines, according to the agency.
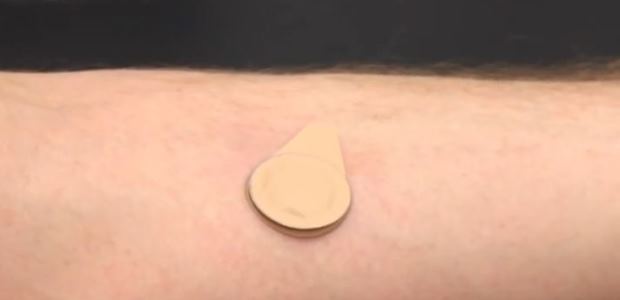
"The microneedle patch under development measures about a square centimeter and is administered with the press of a thumb. The underside of the patch is lined with 100 solid, conical microneedles made of polymer, sugar, and vaccine that are a fraction of a millimeter long. When the patch is applied, the microneedles press into the upper layers of the skin; they dissolve within a few minutes, releasing the vaccine. The patch can then be discarded," CDC's April 27 news release explains.
"Each day, 400 children are killed by measles complications worldwide. With no needles, syringes, sterile water, or sharps disposals needed, the microneedle patch offers great hope of a new tool to reach the world's children faster, even in the most remote areas," said James Goodson, Ph.D., an epidemiologist from CDC's Global Immunization Division. "This advancement would be a major boost in our efforts to eliminate this disease, with more vaccines administered and more lives saved at less cost."
The patch is more stable at varying temperatures than currently available vaccines and takes up less space than the standard vaccine. And because microneedles dissolve in the skin, there is no disposal of needles, thus reducing the risk of accidental needlesticks. The measles patch is expected be manufactured at a cost comparable to the currently available needle and syringe vaccine.
Georgia Tech and CDC’s Global Immunization Division and Division of Viral Diseases recently completed a study that showed the new microneedle patch produces a strong immune response in rhesus macaques. No adverse effects or health issues were noted during the study. This cleared the way for developing proposals for human clinical trials, which could begin as early as 2017.
"We think this collaboration with CDC is an excellent example of how advances in engineering can be used to address important public health problems," said Mark Prausnitz of Georgia Institute of Technology, who served as one of the principal investigators on the study.
For a video about microneedle technology, visit http://youtu.be/wVEF1ckaYEY.