Fourteen Appointed to HHS Tick-Borne Disease Working Group
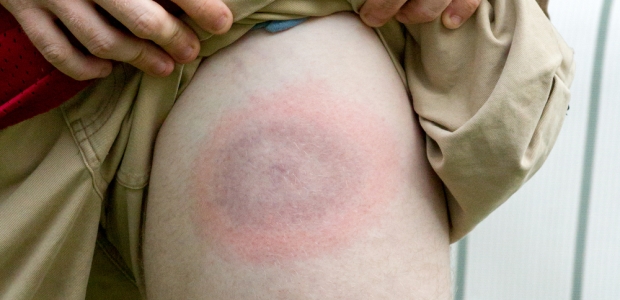
HHS reports that Lyme disease accounts for the majority of tick-borne disease in the United States, and CDC estimates more than 300,000 people are diagnosed with Lyme disease annually, but only about 30,000 of those cases are reported to local and state health departments and to CDC.
Now that the U.S. Department of Health and Human Services has chosen 14 members to serve on its new Tick-Borne Disease Working Group, the panel will hold its first public meetings on Dec. 11 (12:30 to 4:30 p.m. Eastern) and Dec. 12 (9 a.m. to 4:30 p.m.) in Washington, D.C. The panel was established by the 21st Century Cures Act to improve federal coordination of efforts related to tick-borne diseases; the members' job is to review all HHS efforts related to tick-borne diseases, examine research priorities, and identify unmet needs. They are expected to deliver their first report to the HHS secretary and Congress by December 2018.
"We are pleased that the members of the Tick-Borne Disease Working Group are now ready to focus on this important public health issue that affects the lives of hundreds of thousands of Americans each year," said Dr. Don Wright, M.D., MPH, acting assistant secretary for health. "We are impressed by the combined expertise and experience of the working group and are committed to doing everything we can to support its success."
Its members are:
- Wendy Adams, MBA, research grant director for the Bay Area Lyme Foundation
- Dr. John N. Aucott, M.D., assistant professor, Division of Rheumatology, Johns Hopkins University School of Medicine, and director, Johns Hopkins Lyme Disease Clinical Research Center
- Dr. Richard Horowitz, M.D., member, World Health Organization Ad Hoc Committee for Health Equity
- Dr. Lise E. Nigrovic, M.D., MPH, director, Population Health Sciences and Health Services Research Center of the Institutional Centers for Clinical and Translational Research, Boston Children's Hospital, and chair, Pediatric Emergency Medicine Collaborative Research Committee, American Academy of Pediatrics
- Patricia V. Smith, president, Lyme Disease Association
- Karen Vanderhoof-Forschner, LLM, J.D., MBA, co-founder, Lyme Disease Foundation
- Dr. Gary Wormser, M.D., professor of Medicine, Microbiology and Immunology, and Pharmacology, and vice chairman, Department of Medicine, New York Medical College
- Charles Benjamin Beard, Ph.D., acting deputy director, Division of Vector-Borne Diseases, CDC, and associate editor, Emerging Infectious Diseases
- Commander Scott J. Cooper, MMSc, PA-C, U.S. Public Health Service, senior technical advisor and lead officer for Medicare Hospital Health and Safety Regulations, Centers for Medicare and Medicaid Services, HHS
- Dennis M. Dixon, Ph.D., chief, Bacteriology and Mycology Branch, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health
- Kristen Honey, Ph.D., P.M.P., senior policy analyst, Office of Management and Budget; senior research scholar, Stanford University and member, Stanford University Lyme Disease Working Group
- Capt. Estella Jones, D.V.M., director, Medical Countermeasure Regulatory Science and Senior Regulatory Veterinarian, Office of Counterterrorism and Emerging Threats, FDA
- Allen Richards, Ph.D., director, Rickettsial Diseases Research Program, Naval Medical Research Center, U.S. Department of Defense
- Dr. Vanila M. Singh, M.D., M.A.C.M., chief medical officer, Office of the Assistant Secretary for Health, HHS
HHS reports that Lyme disease accounts for the majority of tick-borne disease in the United States, and CDC estimates more than 300,000 people are diagnosed with Lyme disease annually, but only about 30,000 of those cases are reported to local and state health departments and to CDC.