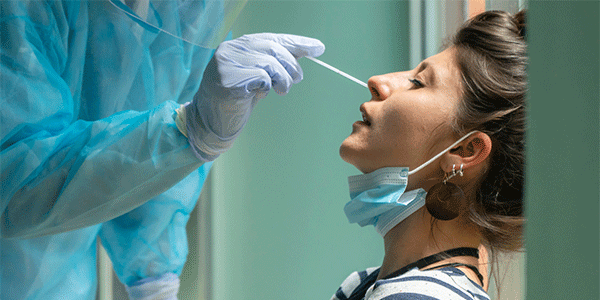
FDA Authorizes First Non-Prescription Home Coronavirus Test
The test delivers results to your smartphone within 15 minutes of collecting a sample.
- By Nikki Johnson-Bolden
- Dec 17, 2020
The FDA authorized the first coronavirus test that can be bought in a store without a prescription on December 15, says NPR.
The at-home test is made by the Australian company Ellume and will be available in January. It is administered using a swab and produces results within 15 minutes via a smartphone app. Collecting the sample for testing takes about five minutes.
“[The] authorization is a major milestone in diagnostic testing for COVID-19,” FDA Commissioner Stephan Hahn said.
The test will cost about $30 and can be used for both adults and children as young as 2-years-old.
About the Author
Nikki Johnson-Bolden is an Associate Content Editor for Occupational Health & Safety.