
Why Do You Need 10% Vol Oxygen to Operate a Catalytic Bead LEL Sensor?
Catalytic bead LEL sensors need a certain level of oxygen to correctly read combustible gas up to 100% LEL.
The catalytic bead lower explosive limit (CB LEL) sensor is widely used for combustible gas detection based on its low cost, ease of use, and the ability to detect a wide range of gases. However, for some special applications, such as environments with less than 10% vol oxygen (O2), the CB LEL sensor is not recommended. Here's why:
Reason #1: 10% vol O2 allows gas readings up to 100% LEL.
To help you better understand this, let me explain the basic principle of how a catalytic bead LEL sensor works. A catalytic bead LEL sensor senses a combustible gas through flameless combustion that occurs with the help of electrically produced heat and a catalyst material coating on the sensing bead. In other words, a CB LEL sensor detects gas through the actual burning of the gas. This is why it can detect a wide range of gases and can detect multiple gases at the same time. Like three elements of a fire, CB LEL gas sensing requires fuel (combustible gas in this case), heat (by a metal wire coil buried in the sensor bead), and oxygen.
How much oxygen is needed for CB LEL gas sensing depends on how much and what type of combustible gas is present in the detection environment. Let's use methane (CH4) gas as an example. When methane burns with oxygen, each CH4 molecule consumes two oxygen molecules in a complete oxidation reaction, resulting in byproducts of one carbon dioxide and two water molecules. The chemical reaction formula is:
CH4 + 2O2 => CO2 + 2H2O
The lower explosive limit (LEL) for methane is 5% vol. In order to burn this 5% vol (or 100% LEL) methane completely, you will need at least 2x5%=10% vol O2 gas. If O2 is less than 10%, you will not be able to read up to 100% LEL methane due to the lack of O2 in the reaction. Different gases may have different LEL numbers and require different ratios of O2 to react. In best practice, to keep the meter readings for most gases up to 100% LEL (typical measurement range of a gas detector), 10% vol O2 is the minimum requirement, allowing the sensor to measure up to this range. This is one reason why we don’t recommend using catalytic bead LEL sensors in an environment with less than 10% vol oxygen.
Reason #2: 10% vol O2 ensures measurement accuracy.
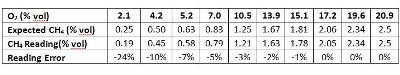
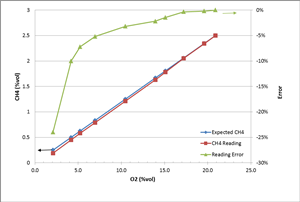
To study how the gas reading changes under different O2 levels, we conducted an experiment. Two gas cylinders, #1 (2.5% vol CH4 balanced with air 20.9% vol O2) and #2 (100% N2), were blended to adjust the O2 gas concentration levels. One thing to keep in mind is that the ratio of CH4 to O2 is kept the same at 2.5/20.9 in this experiment. So in theory, there should be enough O2 to react with CH4. The Ventis® MX4 multi-gas detector (equipped with LEL, O2 and CO sensors) was used to read the blended gas concentration. The table and chart below show the test results.
You can see clearly from the results that the CH4 reading is still accurate enough (error <5%) as long as the O2 level is high enough (over 10%). As the O2 level gets lower and lower, the reading accuracy starts to decrease (reading lower than the real concentration). When the O2 level is at 2.1% vol, the reading error can be as large as 24%. Two reasons explain this deviation. One is that the sensor is calibrated at a condition of 20.9% vol O2. The larger deviation of the O2 level results in a bigger error of the CH4 reading. The other reason is that not all O2 molecules are reaching and impacted by catalyst material on the sensing bead surface when the O2 concentration is so low. Hence, not all the CH4 molecules participate in the chemical reaction. This is why the reading is low. So in order to keep the instrument reading of combustible gas accurate enough, in practice, it's better to operate the LEL sensor in the environment with O2 higher than 10% vol.
What If My O2 is Below 10% Vol?
Should I trust my catalytic bead LEL reading? The answer is, "It depends." It all depends on the gas environment, how much O2 is present, and the gas reading you get on your detector. Let's still use methane as an example. When there is not enough oxygen, the reaction between methane and oxygen becomes complicated. Some gas molecules may still react fully and result in carbon dioxide. Some gas molecules may have incomplete combustion due to the lack of oxygen and result in carbon monoxide as a byproduct. The incomplete chemical reaction formula is:
2CH4 + 3O2 => 2CO + 4H2O
If you understand this, you should feel confident on how to interpret your data and be able to judge whether the reading is trustworthy or not in an environment with less than 10% vol O2. For example, in an environment with 8% vol O2, your LEL sensor reads 10% LEL (or 0.5% vol) methane (CH4). This gas reading might still be accurate (with error less than +/- 5%), as the complete combustion only needs 1% vol O2. There is enough oxygen to burn this amount of methane gas in this particular circumstance.
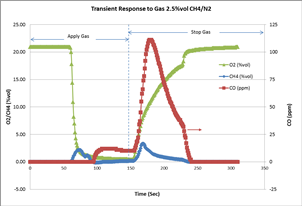
We conducted another experiment to demonstrate how an LEL sensor responds to methane gas in an inert (no oxygen) environment. A 2.5% vol of CH4 balanced with N2 was applied to the MX4 multi-gas detector with LEL, O2, and CO sensors. The transient gas readings were recorded by a specifically designed and built instrument data-logging firmware that can record the gas readings every second. The chart below shows the gas responses when the 2.5% vol CH4/N2 was applied and then stopped after approximately 150 seconds. The residual O2 in the instrument and sensor allows the LEL sensor to still read as high as 2.16% vol before it starts to drop, due to the lack of oxygen in the chemical reaction. When the oxygen becomes lower and lower, carbon monoxide starts to accumulate. This confirms the incomplete combustion of CH4 inside the sensor, as explained above.
Another interesting phenomenon observed in this experiment is that the incomplete combustion happened rapidly when the gas was stopped. During this recovery time, the residue of high concentration CH4 inside the gas environment and O2 diffused from the ambient causing both complete and incomplete combustion reactions. The CO reading jumped to as high as 110 ppm before it dropped slowly to zero. The LEL peak reading was recorded as high as 3.28% vol during this recovery process.
Now let's look at another example. We have 4% vol O2 and our LEL sensor reads 40% LEL (or 2% vol) methane. Is this reading trustworthy? The answer is probably not. In this case, 4% vol O2 only allows complete combustion of methane at no more than 2% vol (or 40% LEL). If the gas concentration is 3% vol (60% LEL), you may still just read 2% vol because there is not enough oxygen to burn all the gas.
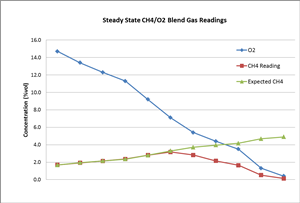
To further investigate this, we designed another experiment: We blended gas cylinder #1 (10% vol CH4 balanced with N2), gas cylinder #2 (100% N2), and gas cylinder #3 (zero air: 20.9% O2). Gas cylinder #1 and #2 were used to adjust the methane gas concentration down to 5% vol (the maximum range the LEL sensor can read). The blended gas was then again blended with cylinder #3. By adjusting the ratio of the blended gases, a steady mixture of CH4/O2 gas can be achieved. An MX4 gas detector was continuously exposed to this mixed gas until a steady reading was recorded for each test level.
The chart below shows that the actual CH4 readings match well with the expected CH4 values when the O2 gas concentration is high enough (over two times of CH4 concentration). The CH4 reading depended on CH4 level in this case. When the O2 concentration was further reduced, the actual CH4 gas readings started to deviate from its true concentration (expected CH4). Further analysis shows the CH4 readings were close to about 50% of the O2 concentration level, as the combustion was determined by O2 level instead of CH4 under this circumstance. A small level of carbon monoxide (CO) was also observed this time, which supports that incomplete combustion occurred.
Summary
In conclusion, catalytic bead LEL sensors need a certain level of oxygen to correctly read combustible gas up to 100% LEL. A low concentration O2 may cause a large reading error and give you low gas readings due to incomplete combustion with possible carbon monoxide as a byproduct or unreacted combustible gas molecules. Therefore, Industrial Scientific does not recommend using catalytic bead LEL sensors in an environment with less than 10% vol O2.
Instead, dilution tubes or infrared (IR) sensors are recommended to use when oxygen levels are less than 10% vol.
This article originally appeared in the August 2018 issue of Occupational Health & Safety.